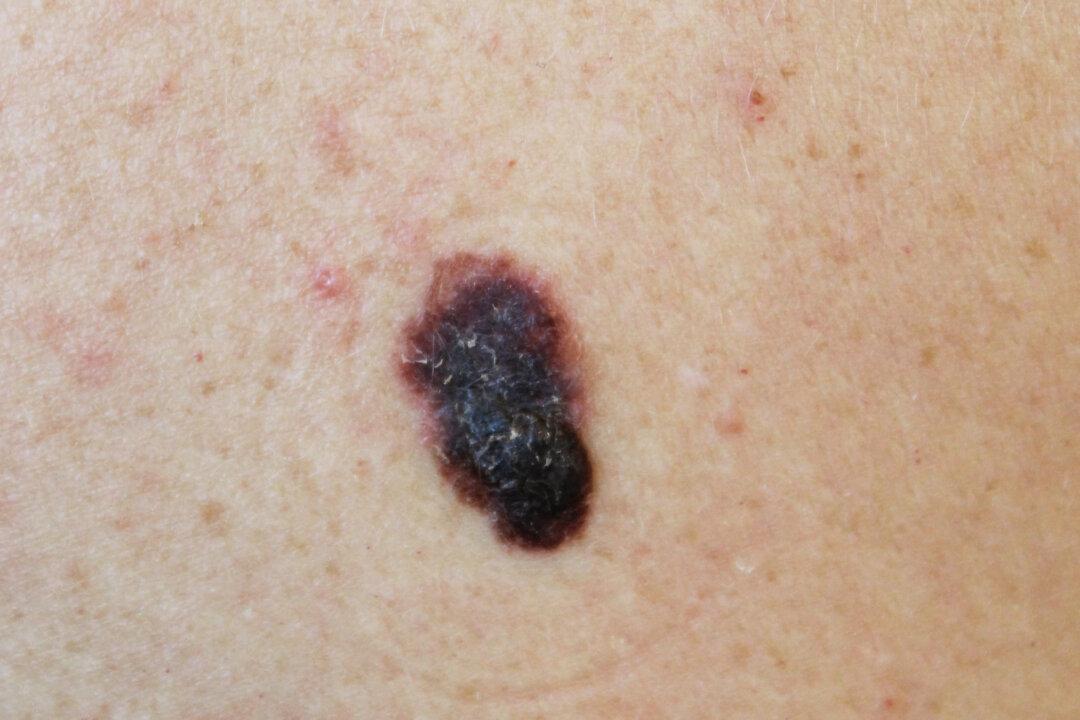
Melanoma cancer patients with solid tumors can turn to a new treatment thanks to the U.S. Food and Drug Administration’s (FDA) approval of a new class of immunotherapy. The new treatment may offer hope to those facing an otherwise deadly stage of disease.
Amtagvi (lifileucel) is the first tumor-infiltrating lymphocyte (TIL) therapy to hit the market. The FDA granted accelerated approval to manufacturer Iovance Biotherapeutics on Friday for the drug’s use to treat patients with advanced melanoma that is unable to be removed with surgery, or that has spread to other parts of the body.