We will now discuss how the LNPs are constructed and how they behave in the body. The engineering of these molecules must keep the capsule stable during transit but also allow it to dissolve quickly once injected.
If the LNPs are too stable, they may move throughout the body to distant organs instead of disintegrating locally at the injection site as intended. Other properties of the LNPs also affect the likelihood of adverse events, such as their electrical charge and their tendency to cluster.
Summary of Key Facts:
- The lipid nanoparticle (LNP) capsule contains the active ingredient messenger RNA (mRNA).
- The LNP is formed by lipids “teaming up” together to form a ball.
- LNP molecules offer great potential as a delivery vehicle, however, the design of the LNP can cause harm.
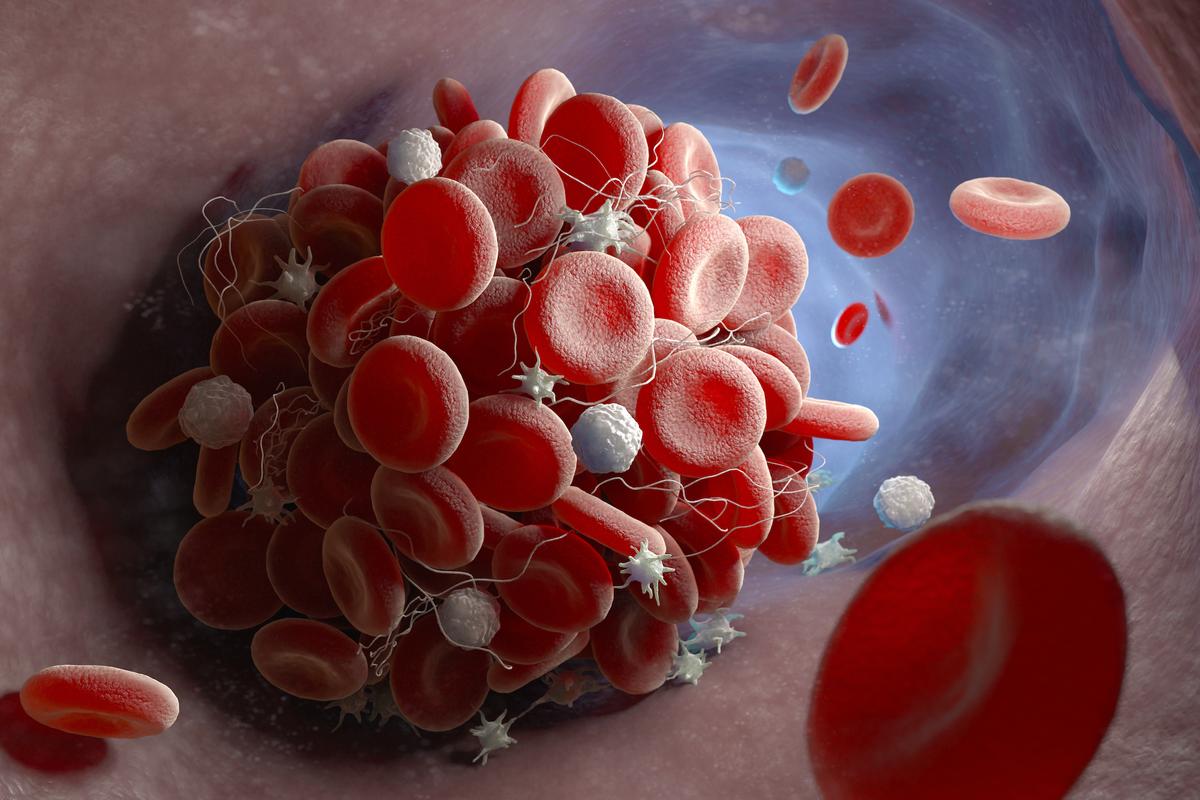
- The LNP capsule can cluster with other LNPs or fall apart after injection, potentially causing clotting.
- If the LNP capsule falls apart, loose strands of mRNA can circulate in the blood.
- Because the mRNA is negatively charged, loose mRNA in the blood can cause clotting if it clusters with positively charged molecules.
- The LNP capsule lipids also have properties that may cause clotting or trigger the immune system to overreact.
- Researchers knew about these possibilities before the vaccines were authorized.
- The regulatory agencies knew about the possibility of harmful effects before they were even injected into the body.
- The possibility of multiple boosters causing harm was also known before authorization.
- As time passes, we are learning more about the possible mechanisms behind these adverse events.