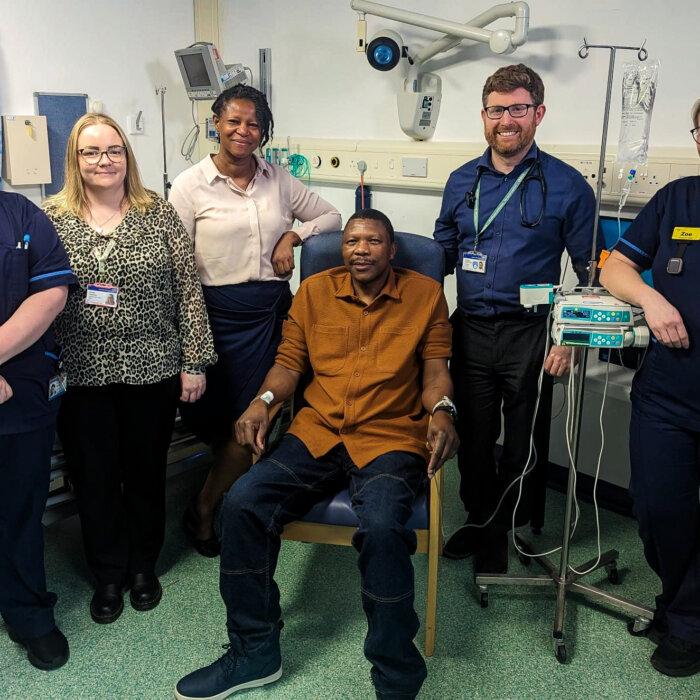
An immunotherapy drug could spare bowel cancer patients the need for surgery and chemotherapy after a clinical trial found it was 100 percent successful in eliminating the disease.
British pharmaceutical giant GSK claimed their drug, Jemperli, also known as dostarlimab, showed “unprecedented results,” with no trace of the disease remaining in any of the 42 patients treated.
Jemperli is already approved by the UK’s National Health Service (NHS) for women with some types of advanced or recurrent cancer of the womb.
Immunotherapy drugs use patients’ own disease-fighting white blood cells to target cancerous tumours, but their cost can be very high and the treatment is not always available on the NHS.
Findings Presented at Chicago Conference
The trial findings were presented at the American Society of Clinical Oncology (ASCO) conference in Chicago over the weekend of June 1–2, where thousands of scientists and pharmaceutical industry representatives gathered.The data showed that all the patients in a trial, led by Memorial Sloan Kettering Cancer Centre in New York City, had a completely successful response to treatment, with no evidence of tumours showing up in scans.
The current NHS recommended protocol for patients with this type of cancer is chemotherapy plus radiation, followed by surgery to remove the tumour, along with portions of the intestine and surrounding tissue.
While chemotherapy can kill cancer cells, it is well known for its serious side-effects, while radiotherapy and surgery also come with risks.
Andrea Cercek, principal investigator for the phase II study, said the new treatment showed “durable complete tumour regression without the need for life-altering treatment” such as chemotherapy and surgery.
She added, “As a clinician, I’ve seen firsthand the debilitating impact of standard treatment of dMMR rectal cancer and am thrilled about the potential of dostarlimab in these patients.”
Hesham Abdullah, a senior vice president at GSK, claimed the trial data was “remarkable” and said that trials were ongoing to evaluate the drug further.
Surging Rates of Bowel Cancers
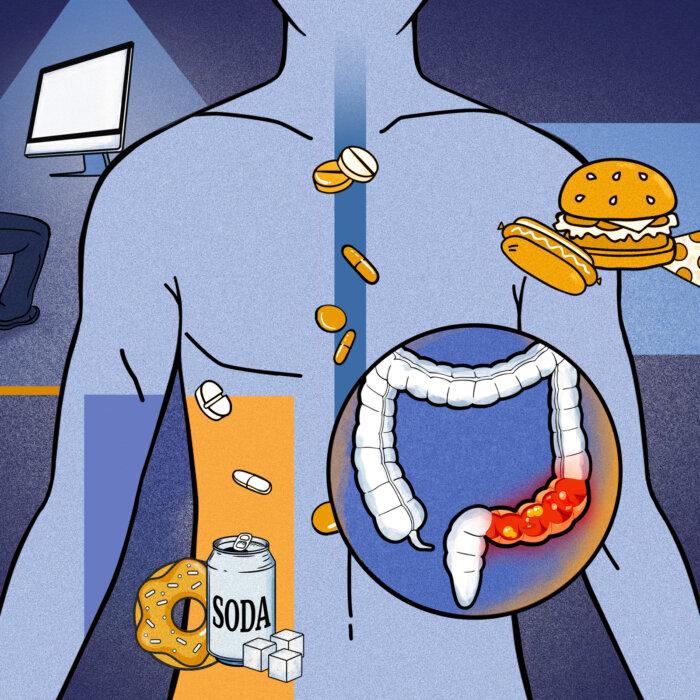
Colorectal cancer is the third most commonly diagnosed type of cancer in the world, with rates surging over the past two decades, especially in younger people.The rate at which people under the age of 50 in the UK are dying from bowel cancer is on course to rise by a third this year, according to projections that experts say are alarming and stem from a surge in obesity, poor diets, and physical inactivity.
According to a study published in the journal Annals of Oncology, UK death rates from bowel cancer in those aged 25 to 49 are predicted to increase by 39 percent in women and 26 percent in men in 2024, compared with the average between 2015 and 2019.
Bowel cancer is the second most common cause of cancer death in the UK, accounting for 10 percent of all cancer deaths.
Scientists predict that bowel cancer death rates will rise in women of all ages in the UK, bucking the trend for improved survival rates in other forms of cancer common in women, such as breast cancer.
Surging rates of obesity, ultra-processed food, so-called “forever chemicals,” and the overuse of antibiotics—which upset the balance of gut bacteria—are all touted as possible factors for the increase in the disease.