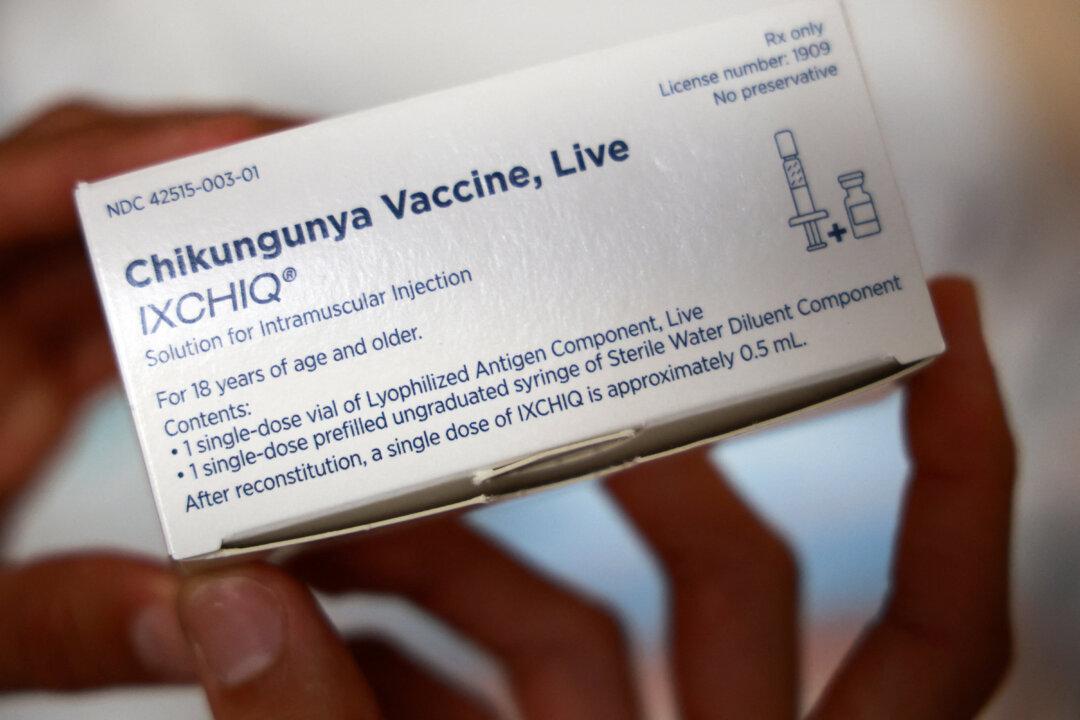
Federal officials paused administration of a vaccine against the chikungunya virus for some adults because post-vaccination problems may be related to the shot, regulatory authorities said on June 5.
Seventeen serious adverse events—including two deaths—had been reported by early May among people who received the chikungunya vaccine.