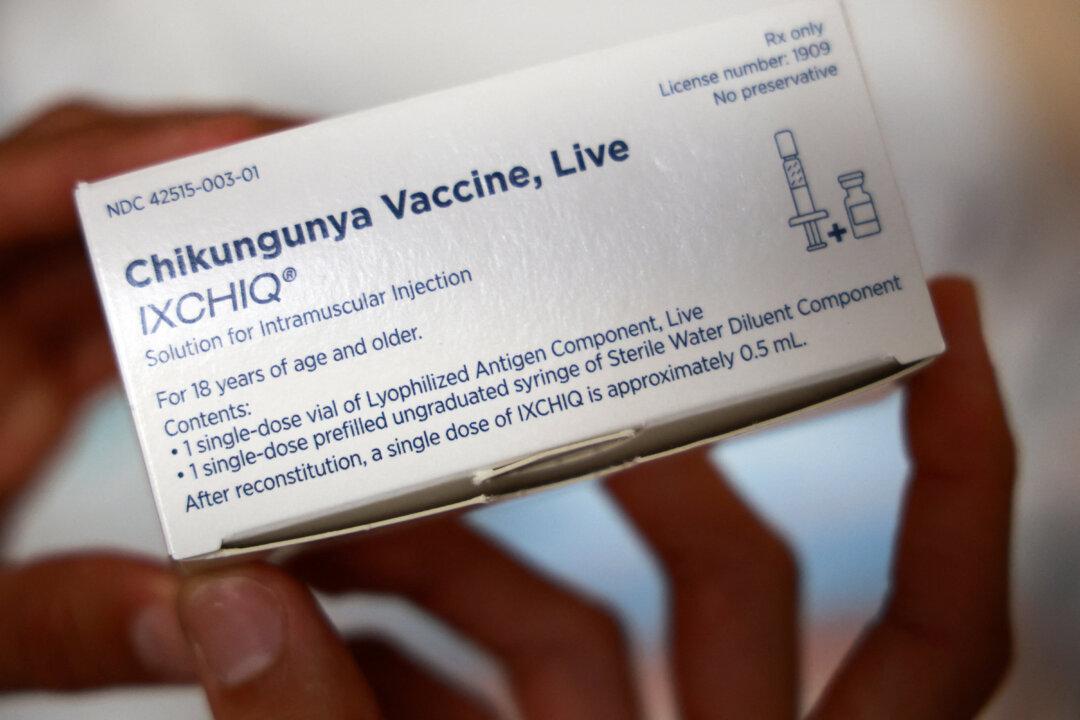
The Food and Drug Administration has lifted its hold on a vaccine against chikungunya, the agency announced on Aug. 6, as it adjusted the label for the shot.
The FDA is ending its recommended pause, which effectively stopped administration of Valneva’s vaccine Ixchiq, the agency said in a statement.