A drug approved as the first breakthrough treatment for a leading cause of blindness has left some patients with the very fate it aimed to prevent, triggering alarm over its unforeseen side effects.
The Potential Impact
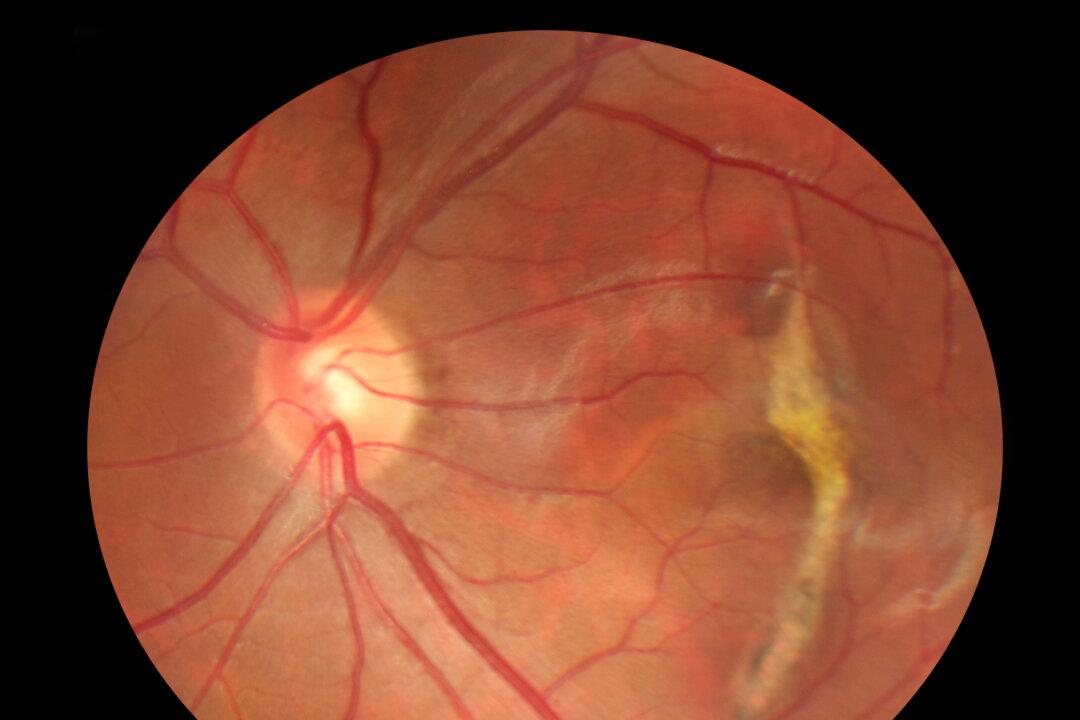
The U.S. Food and Drug Administration granted approval (pdf) in February for a new drug, Syfovre (pegcetacoplan injection), to treat geographic atrophy, an advanced form of dry age-related macular degeneration that can lead to blindness. The drug was initially hailed as a breakthrough in the fight against this previously untreatable condition affecting nearly 1 in 100 Americans over 50.“The approval of Syfovre is the most important event in retinal ophthalmology in more than a decade,” Dr. Eleonora Lad, lead investigator for the OAKS study, sponsored by Apellis Pharmaceuticals, Inc., which manufactures Syfovre, and director of ophthalmology clinical research at Duke University Medical Center, said in a statement at the time.