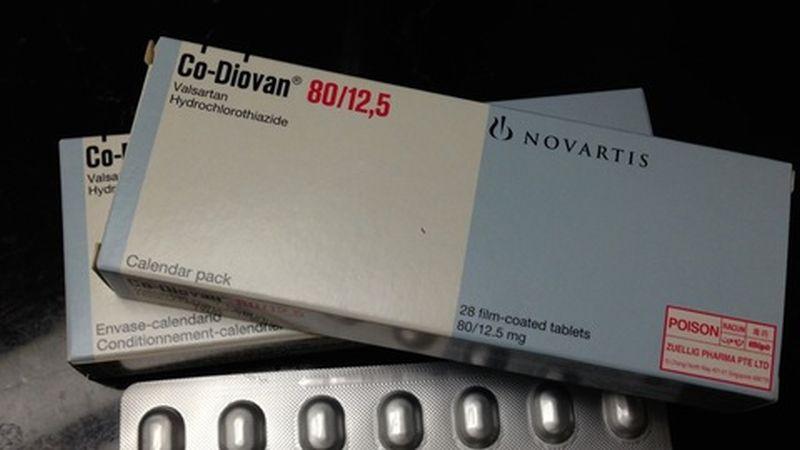
The U.S. Food and Drug Administration (FDA) has released a list of blood pressure medications that are safe to use, meaning they’re free of nitrosamine.
On April 5, the agency posted a list of about 40 blood pressure and heart medicines where nitrosamine, which has been linked to cancer, is “not present.”