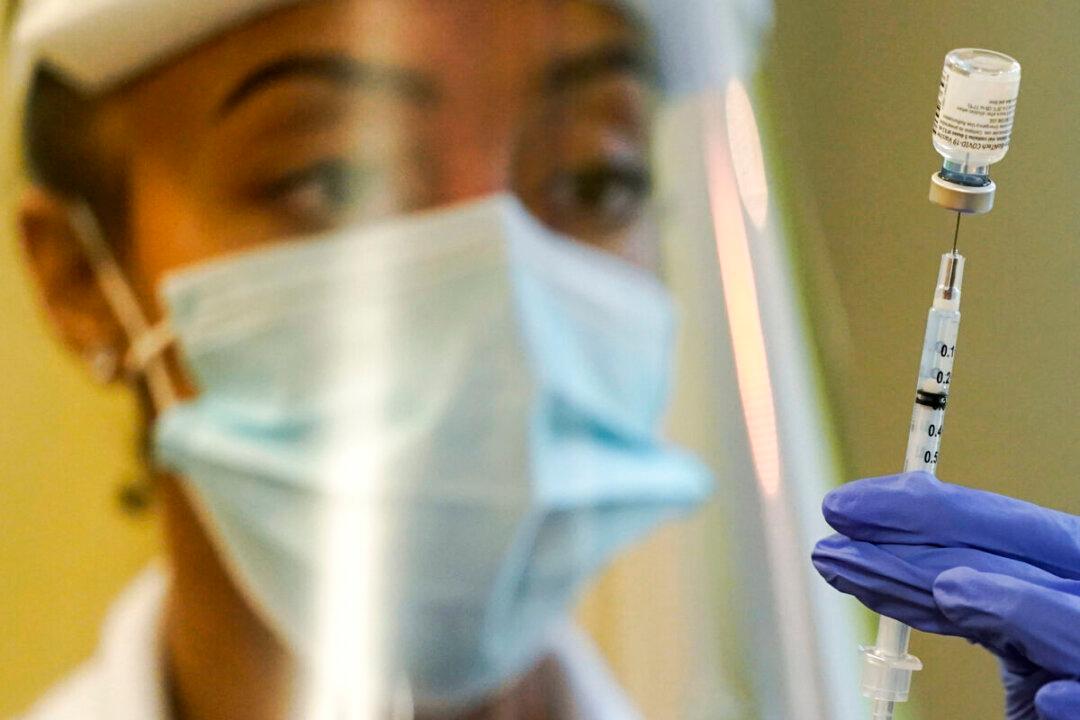
A booster dose of Pfizer-BioNTech’s COVID-19 vaccine five months after a second dose reduces an individual’s risk of hospitalization by 93 percent, according to an Israeli study published on Oct. 28.
The study, published in The Lancet (pdf), was conducted by Israel’s largest HMO, Clalit Health Services, with funding from Harvard Medical School. Researchers compared data from 728,321 people aged 12 and up who had received a booster shot, as well as a similar number of people who received just two doses of Pfizer’s vaccine at least five months prior.