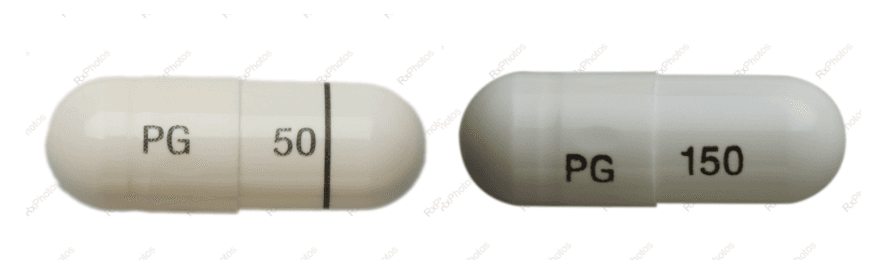
A nationwide recall has been issued for a common pain medication due to dosage issues, Health Canada says.
One lot of bottled JAMP-Pregabalin 50 milligram capsules may mistakenly contain 150 milligram capsules, which could lead to overdose and potentially cause serious or fatal health risks, the May 3 recall notice said.