SHANGHAI—Shares of China’s Zhejiang Huahai Pharmaceuticals Co Ltd tumbled in early trade on Oct. 8, the first day of trading since the U.S. Food and Drug Administration halted imports of drug ingredients or medicines made by the company.
The drug maker’s Shanghai-listed stock fell the maximum allowed 10 percent, its biggest drop in three months, after the company said on Oct. 7 that the move by the U.S. FDA along with regulators in Europe would hit its export business.
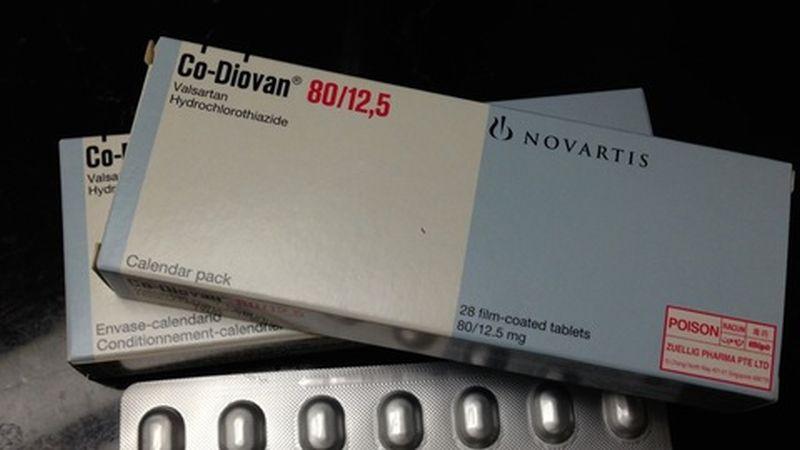
The bulk manufacturer of the high blood pressure treatment valsartan recalled the product from consumers in the United States in July after an impurity linked to cancer was detected.
The case has thrown a spotlight on hidden risks in China’s sprawling market for generic medicines and active pharmaceutical ingredients (APIs), and underscored regulatory blind spots that have caught out Chinese, European and U.S. watchdogs.
European authorities said late last month they had found that Huahai did not comply with good manufacturing practices and that the company’s factory in Linhai, China, was no longer authorized to produce valsartan.
Chinese markets resumed trading on Oct. 8 after a week-long national holiday. Huahai, which says its makes over 50 drugs, APIs and intermediate products, made 61 percent of its total revenue last year outside of China.
Huahai said in a statement on Oct. 7 that it faced risks including being sued by clients and from further action by authorities in Europe. It added that the firm and its units had been sued by U.S. consumers over the valsartan issue.
By Adam Jourdan