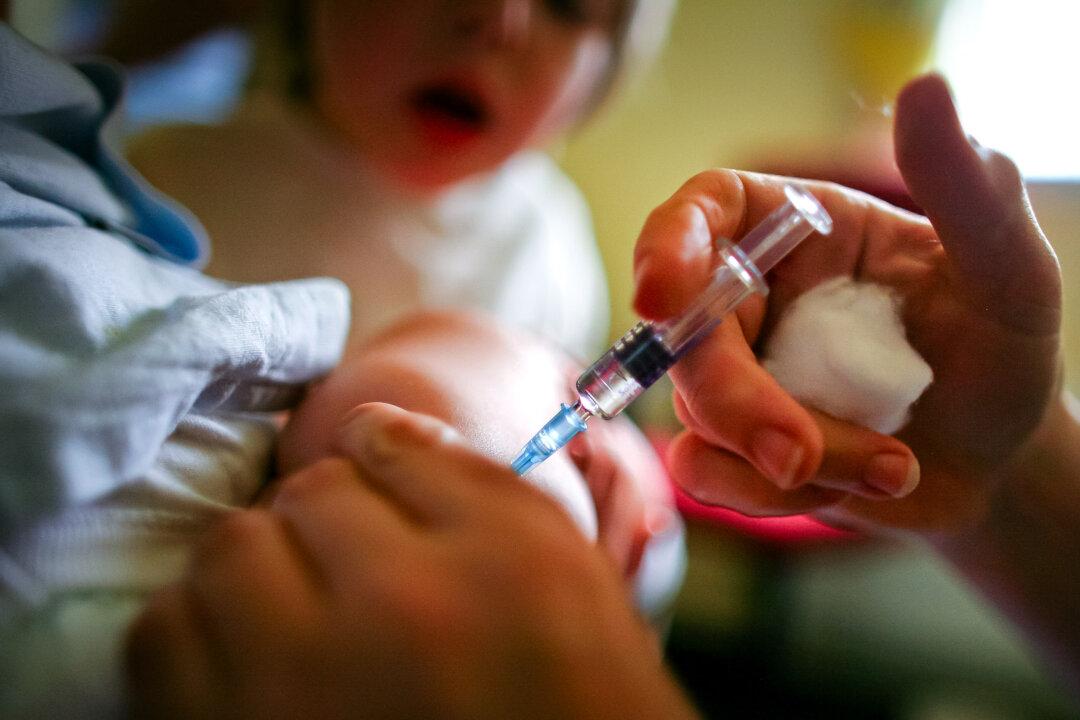
A medical freedom advocate has described Washington state’s effort to change the definition of vaccine to allow novel injections into the state’s universal childhood vaccine purchase program as “another opportunity to enrich public health and pharmaceutical companies without liability or adequate product testing.”
Washington state Senate Bill 5982 is defined in the bill’s language as an act to “update” the definition of vaccine from “a preparation of a killed or attenuated living microorganism, or fraction thereof, that upon administration stimulates immunity that protects against diseases” to simply “an immunization.”