Tylenol Recall Expands; Includes Products Sold in US
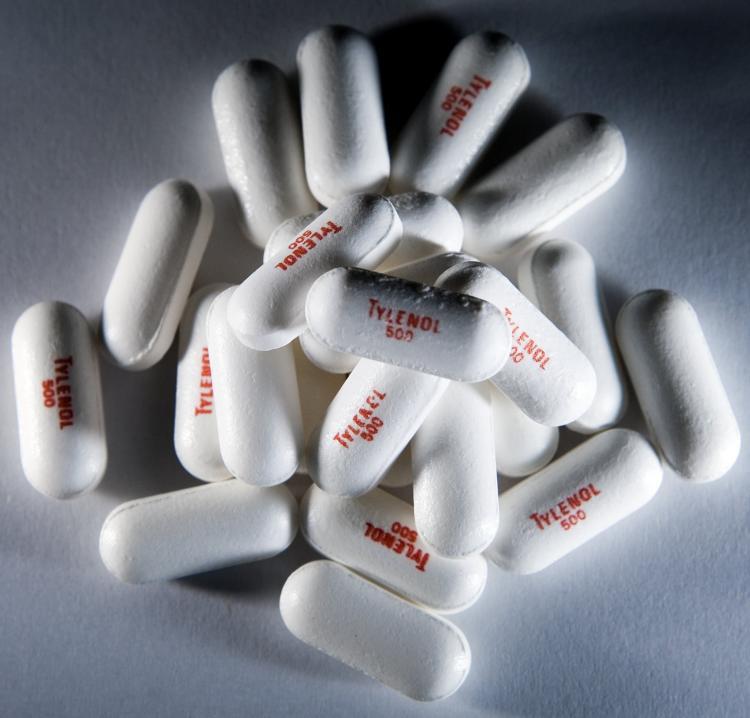
Tylenol makers’ recall of over-the-counter medicines has expanded to include 21 additional lots of Tylenol products.
Extra Strength Tylenol is displayed. The FDA has investigated consumer reports of serious side effects since the Tylenol recall and other children's drugs manufactured by McNeil Consumer Healthcare. Brendan Smialowski/Getty Images
|Updated: