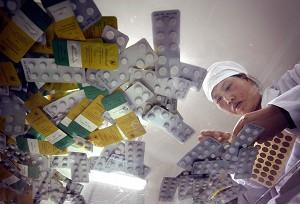
On August 8 2007, Guangzhou City Intermediate People’s Court held criminal proceedings of a well-known counterfeit medicine manufacturing case. Five people from Qiqihar No.2 Pharmaceutical Co., Ltd, were criminally charged with, “causing a major liability accident.”
The well-known pharmaceutical manufacturer Qiqihar No.2 Pharmaceutical Co., Ltd. held a national GMP (Good Manufacturing Practice; drug production quality control standard) certification. Its pharmaceuticals were sold all over the country.
The five people on trial included one general manager, two deputy general managers, one laboratory director and one purchaser. The people concerned control the company’s purchasing, quality control, sales and production.
According to a Chinese report on Xkb.com.cn, dated August 9, once in court the five defendants exposed a lot of inside information detailing counterfeit medicine production:
1. Purchasing Division – “Cannot Understand Quality Control Report from Vendor”
Mr. Niu Zhongren, the only individual responsible for purchasing raw materials, was only a junior high school graduate. He cited being “unbearably busy,” and replaced the mandatory “field inspections” and “sample testing” with simple “phone calls” in the purchasing process. Mr. Niu believed that drug production quality control was a matter for the Quality Control Division, so it did not fall within his scope of responsibilities. He also claimed that he “could not understand the quality control reports from vendors.”
2. Approval Link – “GMP Certification Was Bought”
Mr. Guo Xingping was the deputy-general manager of the company. He was in charge of purchases, storage and distribution. The prosecution questioned him about why GMP certification requirements were not met. He was asked why in the absence of field investigations and sample testing was the company allowed to purchase drug supplies.
Answering the question, Mr. Xingping used an analogy, “This is like buying pork. If you go to buy two catties of pork (one catty equals 1.1 pounds) but you suspect that the pork contains clenobuterol hydrochloride (a banned substance). Would you go to a pig farm to do the field investigation?” As for the GMP certificate, Mr. Guo claimed that the certification, that was supposed to be approved by the regulating body, the Provincial Medicine Regulation Department and registered in the National Medicine Regulation Department, was purchased by the company. “It is just a disc that contains all the documents. The company bought it for 100,000 yuan (USD$13,150)! It is completely impractical for operating in real life” said Mr. Guo.
3. Laboratory Testing Stage
“Of the eleven laboratory technicians, there are only a few who actually have the relevant industry knowledge. Most people are not trained” Ms Chen Guifen was the Laboratory Director of the company.When she tested a batch of “propylene glycol,” she found the relative density of the batch was not acceptable. However, she did not perform more lab tests to further identify the problem. She wrote false inspection reports following the instructions of Deputy General Manager Zhu Chuahua, who was in charge of quality control.
Ms Chen said, “Of the eleven laboratory technicians, there are only a few who actually have the relevant industry knowledge. Most people are not trained. Many did not even have work permits.”
4. Production and Quality Control Division
“It is the company’s unspoken rule that if raw materials are substandard, we need to approve them (by Inspection Report)”. Deputy General Manager Zhu Chuahua was in charge of the Quality Control Division.
He knew the batch of propylene glycol was a counterfeit product, and that its relative density was not acceptable. In addition, the company’s facilities were ill equipped and the lab technicians were not technically qualified. However, he told Ms Guo to file false inspection reports.
Mr. Zhu said, “It is the company’s unspoken rule that if raw materials are substandard, we need to approve them (by way of an Inspection Report)”. He added, “This is the way it has always been done”.
5. The Final Comment
“When raw materials are first received, employees are each responsible for their specific task. I don’t necessarily know what that might be .”As the general manager of the company, Mr. Yin Jiade denied his responsibility; even though he knew most of his staff were not technically qualified. Mr. Yin said, “When raw materials are first received employees are each responsible for their specific task. I don’t necessarily know what that might be.”
According to the report, 13 people in Guangzhou have died because of the medicine and dozens of victims want to file claims for more than 20 million yuan (US$2,630,129) in damages.