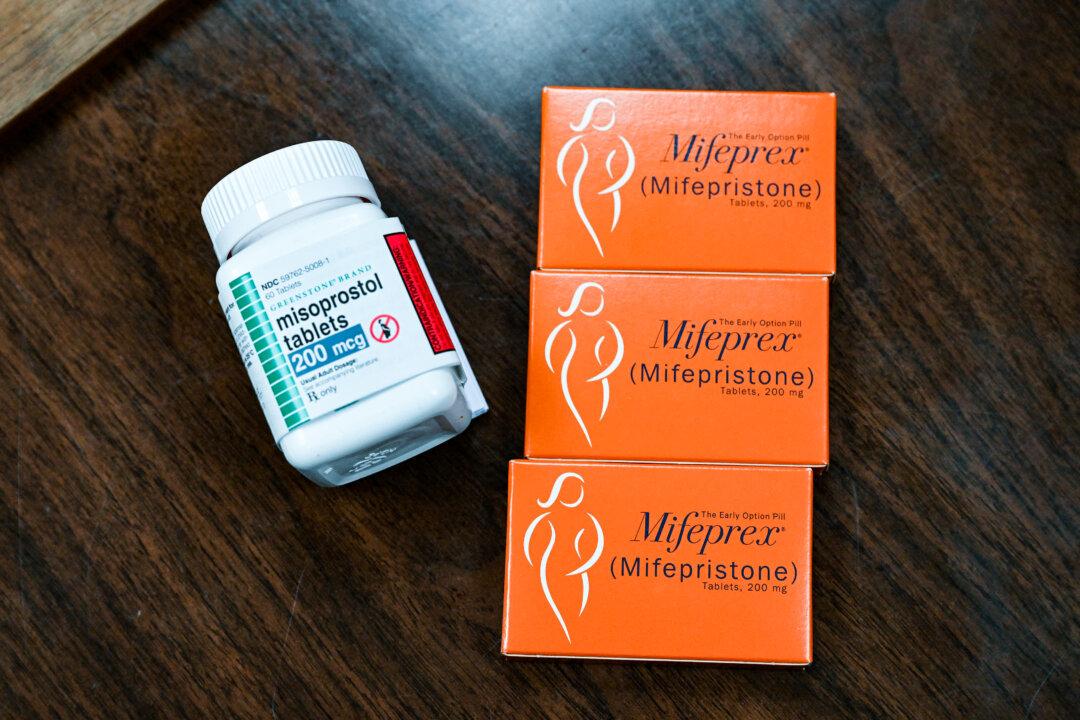
The U.S. Supreme Court decided Friday that mifepristone, an abortion pill, will be broadly accessible amid an ongoing legal battle over its regulatory approval.
Mifepristone, approved in 2000 by the Food and Drug Administration (FDA), is part of a chemical abortion process, and is generally taken with another drug called misoprostol to kill an unborn child in pregnancies up to 10 weeks. It is also sometimes used for women who have miscarriages.