In the 21st century, scientists are again introducing the topic of alchemy. Three researchers from Pennsylvania State University have recently shown that particular combinations of elemental atoms have electronic signatures that mimic those of other elements.
“The findings could lead to much cheaper materials for widespread applications such as new sources of energy, methods of pollution abatement, and catalysts on which industrial nations depend heavily for chemical processing,” said lead researcher A. Welford Castleman Jr., Eberly Distinguished Chair in Science, and Evan Pugh, who is a professor in the departments of Chemistry and Physics, in a press release.
The researchers demonstrated that the atoms that have been identified up to now as being imitated can be predicted by looking at the periodic table. The team applied advanced experimentation and theory to quantify these new and surprising findings.
“We’re getting a whole new perspective of the periodic table,” said Castleman.
The team’s findings were published in the Dec. 29 online issue of the journal Proceedings of the National Academy of Sciences.
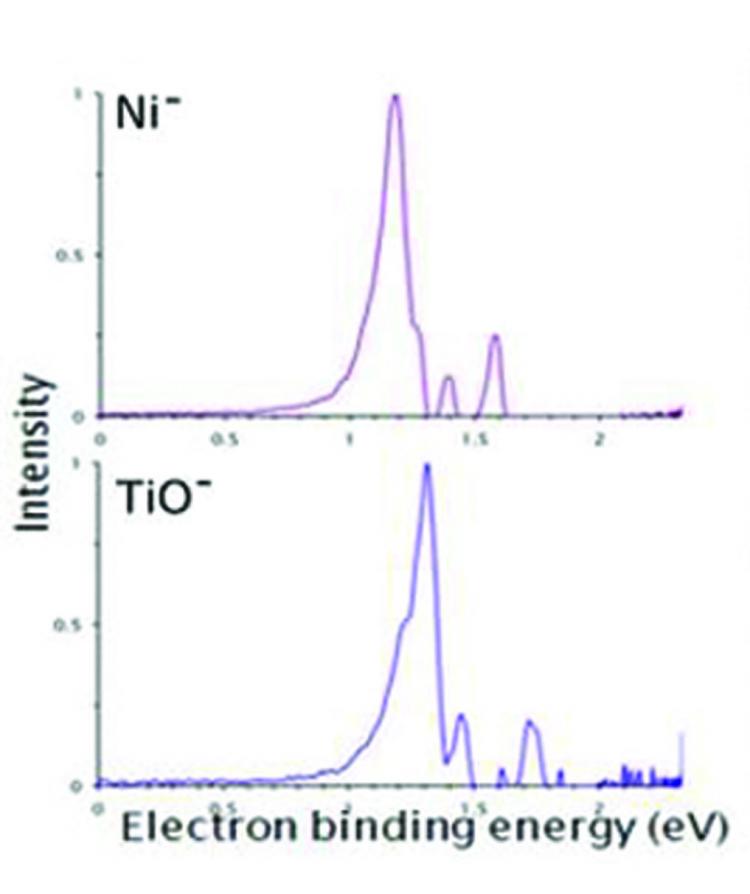
Castleman and his team used a technique known as photoelectron imaging spectroscopy to examine similarities between titanium monoxide and nickel, zirconium monoxide and palladium, and tungsten carbide and platinum.
“Photoelectron spectroscopy measures the energy it takes to remove electrons from various electronic states of atoms or molecules, while simultaneously capturing snapshots of these electron-detachment events with a digital camera,” said Castleman.
“The method allows us to determine the binding energies of the electrons and also to observe directly the nature of the orbitals in which the electrons resided before they were detached.”
They found that the amount of energy required to remove electrons from a titanium-monoxide molecule is the same as that required to remove electrons from a nickel atom. The same is true for the systems of zirconium monoxide and palladium, and tungsten carbide and platinum.
Therefore, the molecules titanium monoxide, zirconium monoxide, and tungsten carbide are superatoms—clusters of atoms that exhibit some property of elemental atoms—of nickel, palladium, and platinum, respectively.
Castleman said that such similarities between atoms and superatoms can potentially save us a lot of money.
“Platinum is used in nearly all catalytic converters in automobiles, but it is very expensive,” said Castleman.
“In contrast, tungsten carbide, which mimics platinum, is cheap. A significant amount of money can be saved if catalytic-converter manufacturers are able to use tungsten carbide instead of platinum. Likewise, palladium is used in certain combustion processes, yet it is mimicked by zirconium monoxide, which is less expensive by a factor of 500. Our new findings are exciting from both a scientific as well as a practical point of view.”
Castleman said that he does not know if the pattern will occur across the entire periodic table. At present, he and his team are working through the transition-metal atoms.
In the future, they are planning to take the research a step further to investigate whether or not the superatoms are chemically similar to their respective single atoms.
“The findings could lead to much cheaper materials for widespread applications such as new sources of energy, methods of pollution abatement, and catalysts on which industrial nations depend heavily for chemical processing,” said lead researcher A. Welford Castleman Jr., Eberly Distinguished Chair in Science, and Evan Pugh, who is a professor in the departments of Chemistry and Physics, in a press release.
The researchers demonstrated that the atoms that have been identified up to now as being imitated can be predicted by looking at the periodic table. The team applied advanced experimentation and theory to quantify these new and surprising findings.
“We’re getting a whole new perspective of the periodic table,” said Castleman.
The team’s findings were published in the Dec. 29 online issue of the journal Proceedings of the National Academy of Sciences.
Castleman and his team used a technique known as photoelectron imaging spectroscopy to examine similarities between titanium monoxide and nickel, zirconium monoxide and palladium, and tungsten carbide and platinum.
“Photoelectron spectroscopy measures the energy it takes to remove electrons from various electronic states of atoms or molecules, while simultaneously capturing snapshots of these electron-detachment events with a digital camera,” said Castleman.
“The method allows us to determine the binding energies of the electrons and also to observe directly the nature of the orbitals in which the electrons resided before they were detached.”
They found that the amount of energy required to remove electrons from a titanium-monoxide molecule is the same as that required to remove electrons from a nickel atom. The same is true for the systems of zirconium monoxide and palladium, and tungsten carbide and platinum.
Therefore, the molecules titanium monoxide, zirconium monoxide, and tungsten carbide are superatoms—clusters of atoms that exhibit some property of elemental atoms—of nickel, palladium, and platinum, respectively.
Castleman said that such similarities between atoms and superatoms can potentially save us a lot of money.
“Platinum is used in nearly all catalytic converters in automobiles, but it is very expensive,” said Castleman.
“In contrast, tungsten carbide, which mimics platinum, is cheap. A significant amount of money can be saved if catalytic-converter manufacturers are able to use tungsten carbide instead of platinum. Likewise, palladium is used in certain combustion processes, yet it is mimicked by zirconium monoxide, which is less expensive by a factor of 500. Our new findings are exciting from both a scientific as well as a practical point of view.”
Castleman said that he does not know if the pattern will occur across the entire periodic table. At present, he and his team are working through the transition-metal atoms.
In the future, they are planning to take the research a step further to investigate whether or not the superatoms are chemically similar to their respective single atoms.