Graphene may be one of the strongest materials on the planet, but a new study raises questions about the limits of using it in the real world.
When material scientists measured the fracture toughness of imperfect graphene for the first time, they found it to be somewhat brittle.
While it’s still very useful, graphene is really only as strong as its weakest link, which they determined to be “substantially lower” than the intrinsic strength of graphene.
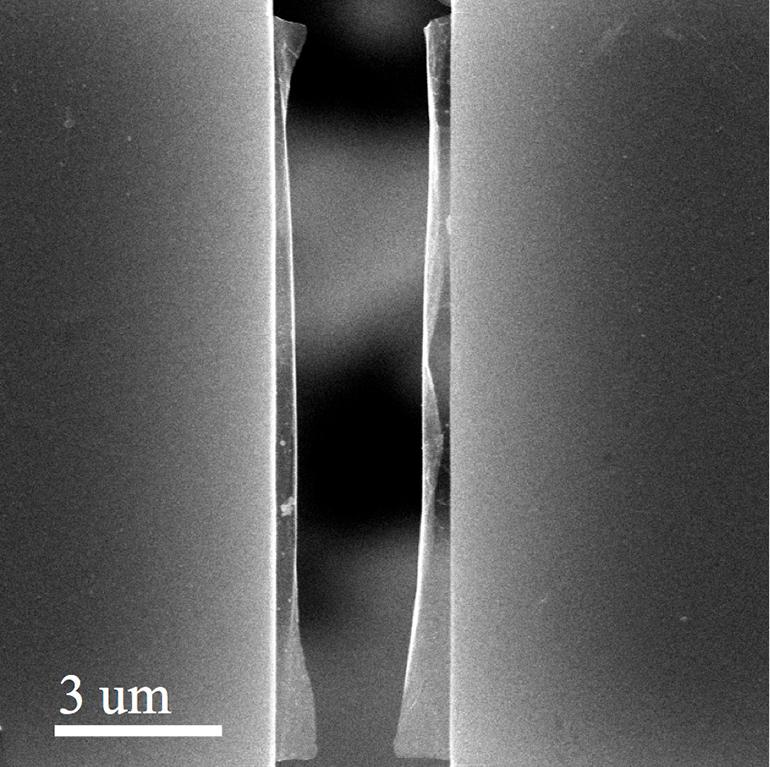
A pre-cracked sheet of graphene was suspended and pulled apart. (The Nanomaterials, Nanomechanics and Nanodevices Lab/Rice University)
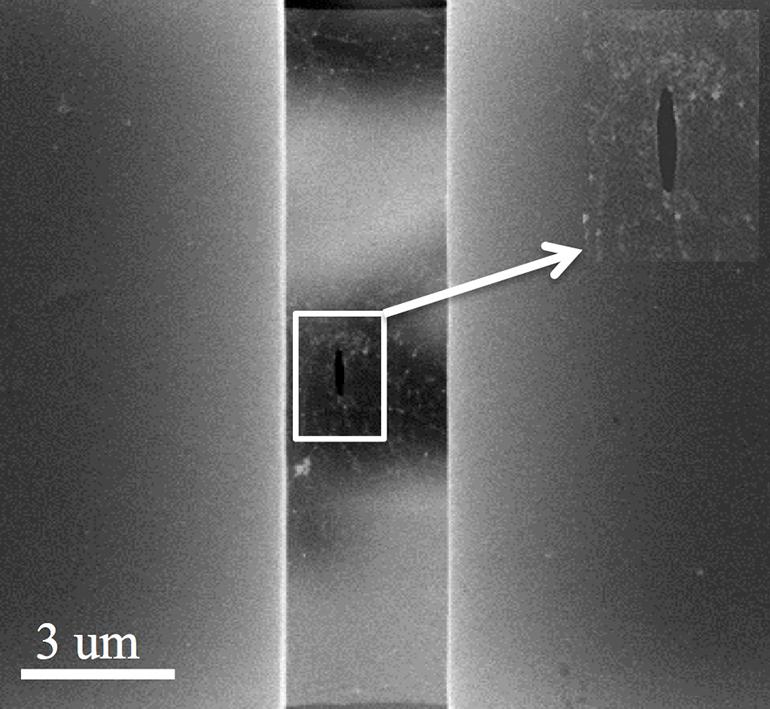
An electron microscope image shows a pre-crack in a suspended sheet of graphene used to measure the overall strength of the sheet. (The Nanomaterials, Nanomechanics and Nanodevices Lab/Rice University)
“Graphene has exceptional physical properties, but to use it in real applications, we have to understand the useful strength of large-area graphene, which is controlled by the fracture toughness,” says Ting Zhu, an associate professor at the Georgia Institute of Technology.
Zhu and Jun Lou, an associate professor at Rice University, report in the journal Nature Communications the results of tests in which they physically pulled graphene apart to see how much force it would take. Specifically, they wanted to see if graphene follows the century-old Griffith theory that quantifies the useful strength of brittle materials.
It does, Lou says. “Remarkably, in this case, thermodynamic energy still rules.”
Perfect Versus Imperfect
Imperfections in graphene drastically lessen its strength—with an upper limit of about 100 gigapascals (GPa) for perfect graphene previously measured by nanoindentation—according to physical testing at Rice and molecular dynamics simulations at Georgia Tech.
That’s important for engineers to understand as they think about using graphene for flexible electronics, composite material, and other applications in which stresses on microscopic flaws could lead to failure.
The Griffith criterion developed by a British engineer during World War I describes the relationship between the size of a crack in a material and the force required to make that crack grow. Ultimately, A.A. Griffith hoped to understand why brittle materials fail.
Graphene, it turns out, is no different from the glass fibers Griffith tested.
“Everybody thinks the carbon-carbon bond is the strongest bond in nature, so the material must be very good,” Lou says. “But that’s not true anymore, once you have those defects. The larger the sheet, the higher the probability of defects. That’s well known in the ceramic community.”
A defect can be as small as an atom missing from the hexagonal lattice of graphene. But for a real-world test, the researchers had to make a defect of their own—a pre-crack—they could actually see.
“We know there will be pinholes and other defects in graphene,” he says. “The pre-crack overshadows those defects to become the weakest spot, so I know exactly where the fracture will happen when we pull it.
“The material resistance to the crack growth—the fracture toughness—is what we’re measuring here, and that’s a very important engineering property,” he adds.
Additional researchers from Rice, Georgia Tech, Nanyang Technological University in Singapore, and at Tianjin Polytechnic University in China collaborated on the project, which received support from Welch Foundation, the National Science Foundation, the US Office of Naval Research, and the Korean Institute of Machinery and Materials.
Source: Rice University. Republished from Futurity.org under Creative Commons License 3.0.
*Image of “graphene“ via Shutterstock




