Singapore has approved a second oral antiviral drug for treating COVID-19.
Molnupiravir, branded as Lagevrio, was granted interim authorization under the Pandemic Special Access Route (PSAR) by the Health Sciences Authority (HSA) on April 19.
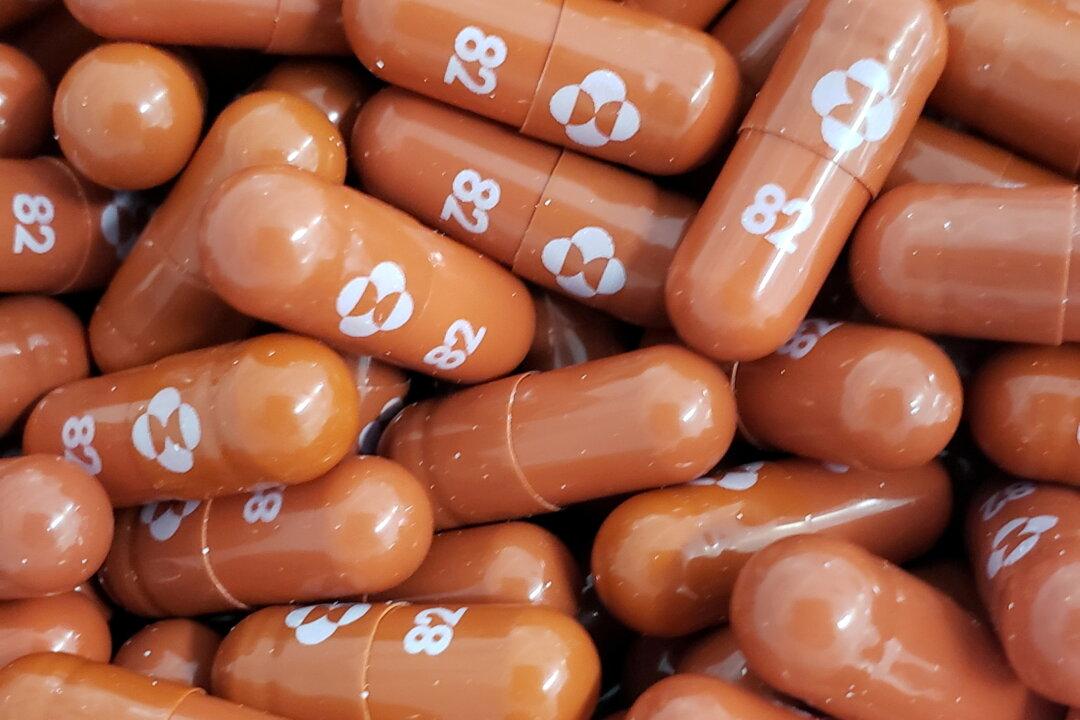
Singapore has approved a second oral antiviral drug for treating COVID-19.