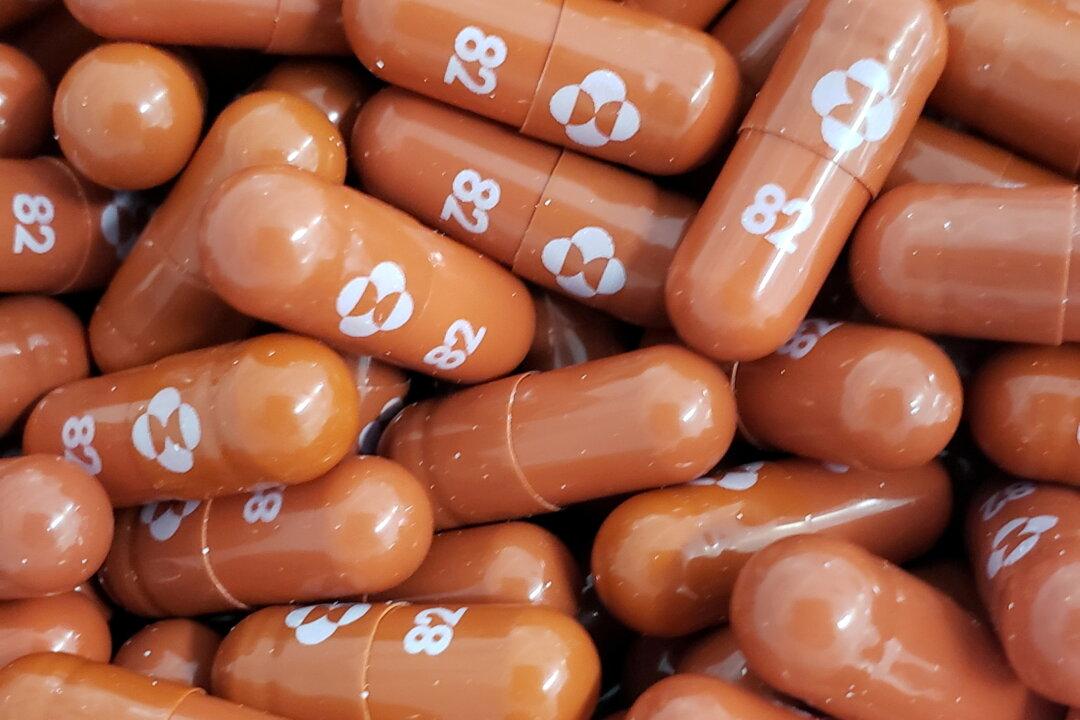
Indian multinational pharmaceutical company Dr. Reddy’s Laboratories will be launching a generic version of Merck’s COVID-19 oral antiviral medication molnupiravir at an extremely affordable treatment rate of 1,400 Rupees ($18.84), 37 times cheaper than in the United States.
Dr. Reddy’s “Molflu” will be priced at $0.47 per 200-milligram pill, with a treatment consisting of a five-day course of 40 pills, 800 mg twice per day. In the United States, the treatment with Merck’s capsules costs $700.