Orange juice, both delicious and nutritious, is enjoyed by millions of people across the world every day. However, new research indicates that it could have potential far beyond the breakfast table. The chemicals in orange peel could be used as new building blocks in products ranging from plastics to paracetamol—helping to break our reliance on crude oil.
Today’s society is totally reliant on the chemicals and materials that are obtained from our diminishing supply of fossil fuels. As such, there is an increasing global focus on the development of renewable chemical feedstocks from a variety of sustainable sources such as sugarcane and fatty acids in the production of biofuels. And the chemically rich essential oils contained within waste citrus peels are another such source that is being investigated with real zest.
This is promising, as the orange juice industry uses highly inefficient and wasteful juicing processes, with almost 50 percent of the fruit thrown away. This gives a real opportunity, then, to develop a sustainable supply of chemicals from the diverse and plentiful molecules locked within the peels.
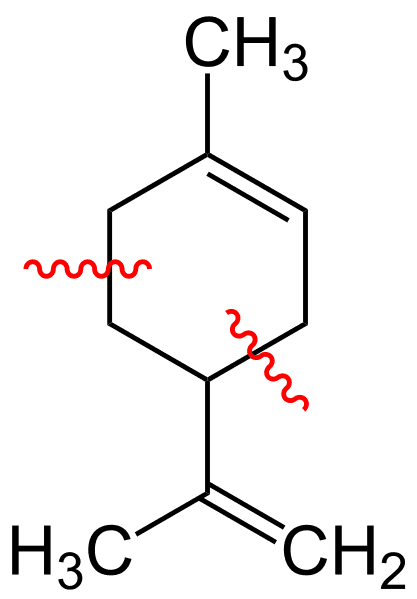
Limonene—A Versatile Building Block
Recent figures estimate around 20 million tons of citrus is wasted each year. As some 95 percent of the oil extracted from these waste rinds is made up of limonene molecules, a colorless liquid hydrocarbon known as C10H16, this waste could yield around 138,000 tons of limonene a year.
Current extraction methods rely on distillation, passing steam through the waste solids, and simply collecting the resulting oil. But recently, researchers at the University of York began investigating microwave extraction techniques as a greener alternative. The team simply placed orange peel and an organic solvent into a microwave and heated for 30 minutes. Within the peel, the water molecules start to boil, rupturing the cells and allowing the limonene to leach out. The results are favorable; the process is much faster, less energy intensive, produces a higher quality of limonene, and in a yield twice as good as conventional methods.