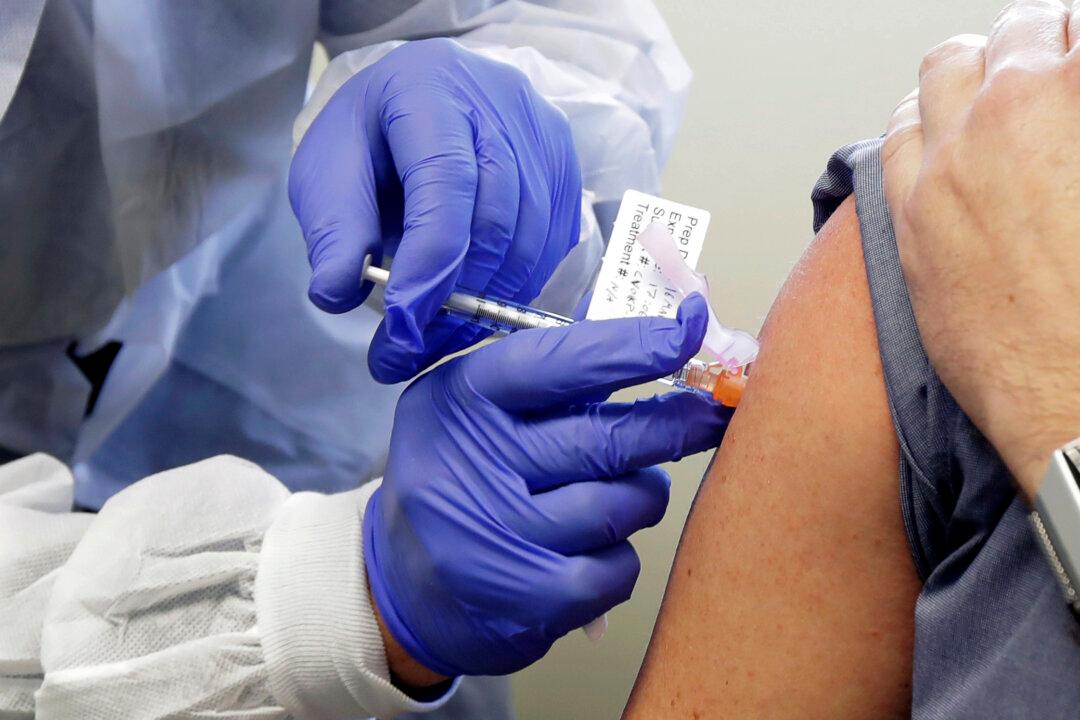
A vaccine against the CCP (Chinese Communist Party) virus provokes an immune system response in older people, Massachusetts-based Moderna announced Wednesday.
A phase 1 trial showed the vaccine produced “consistently high levels” of neutralizing antibodies, or defense against the new illness, in older adults, with similar levels in groups older and younger than 55 years old, Moderna said in a statement to news outlets.