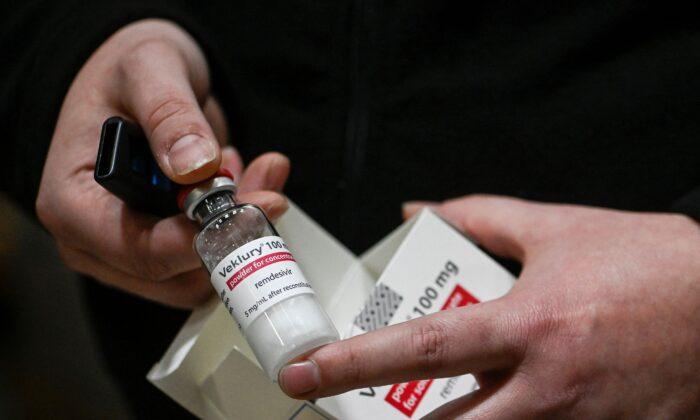
Some nonhospitalized patients seeking treatment for COVID-19 can now use the antiviral remdesivir after U.S. drug regulators on Jan. 21 expanded approval of the drug.
No other research was cited by the FDA.
The panel recommended remdesivir be made an option for nonhospitalized patients alongside the already-available monoclonal antibody treatment sotrovimab from GlaxoSmithKline.
Monoclonal antibodies require a one-time intravenous infusion, versus IV administration across three straight days for remdesivir.
Dr. David Boulware, an infectious diseases specialist at the University of Minnesota who studies COVID-19 treatments, said in a social media post that the Gilead study was small—562 people were enrolled, about half of whom received a placebo—and that a follow-up randomized controlled study would likely find a lower effectiveness against hospitalization.
The FDA’s move means individuals 12 or older who weigh at least 88 pounds, have tested positive for COVID-19, are not hospitalized, and are deemed at high risk for progression to severe COVID-19 can now get the drug, which was previously restricted to hospitalized patients.
The FDA also authorized the drug for children younger than 12 who weigh at least 3.5 kilograms (7.7 pounds) and meet the same criteria.
“Today’s actions provide adults and pediatric patients, with mild-to-moderate COVID-19 who are at high risk of severe COVID-19, with a treatment option they could receive outside of a traditional inpatient hospital setting, including at skilled nursing facilities, home healthcare settings and outpatient facilities such as infusion centers,” Dr. Patrizia Cavazzoni, director of the FDA’s Center for Drug Evaluation and Research, said in a statement.
The expanded clearance “provides FDA imprimatur on what is already happening to fill in the supply limitations of other therapeutics. So, it is a good thing,” Dr. David Wohl, a professor at the University of North Carolina at Chapel Hill’s Institute of Global Health and Infectious Diseases, told The Epoch Times in an email.
Additional studies have also shown “remdesivir is also effective in the early stages of COVID-19 infection, in addition to helping patients who are hospitalized with the disease,” Gilead CEO Daniel O'Day said in a statement.
People who are planning to get the medication, also known as Veklury, should tell their health care provider if they have kidney or liver problems before receiving it, according to the label.



Friends Read Free