U.S. officials are “confident” a CCP virus vaccine will be ready by the end of this year, President Donald Trump said.
“We are very confident that we’re going to have a vaccine at the end of the year,” Trump said during a virtual town hall on May 3. “We think we’re going to have a vaccine by the end of this year and we’re pushing very hard.
“Now, the doctors would say, ‘Well, you shouldn’t say that.’ I’ll say what I think. I’ve met with the heads of the big companies, these are great companies,” he added.
“Yeah, I think we’re going to have a vaccine much sooner rather than later.”
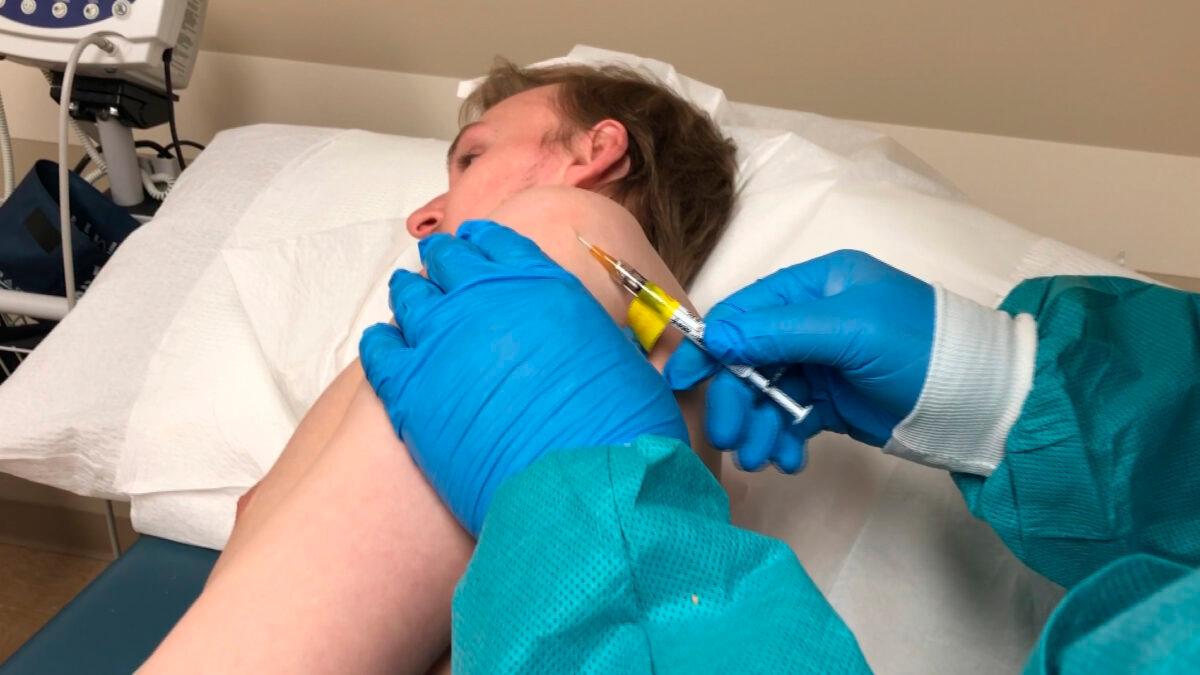
Vaccines are studied in three phases of trials to determine their safety and effectiveness. Each stage involves injecting more people.
Operation Warp Speed
A Trump administration effort called Operation Warp Speed is focusing on having 300 million vaccine doses ready by January 2021.Dr. Anthony Fauci, part of the White House coronavirus task force, said phase 2 trials will show whether candidates are safe and effective. If results are positive, then the administration will work with manufacturers to start scaling up production even as testing continues.
“You, at risk, proactively start making it assuming it’s going to work. If it does, then you can scale up and hopefully get to that timeline,” Fauci, the head of the National Institute for Allergy and Infectious Disease, said on NBC’s “Today” show last week. “I think that is doable if things fall in the right place.”
Fauci in January said it would take 12 to 18 months to develop a vaccine.
Trump told reporters at the White House that he’s in charge of the operation.

Vaccine Production
Johnson & Johnson, Moderna, and other U.S.-based companies are among those working on vaccines, but researchers in other countries are also testing candidates.Asked during the town hall whether another country could have a vaccine ready before the United States, the president said he isn’t worried.
“I don’t care. I just want to get a vaccine that works. I really don’t care. If it’s another country, I’ll take my hat off to them,” he said.
A therapeutic, or an existing drug that works against COVID-19 would be better, he added.
While hydroxychloroquine, remdesivir, and other existing or experimental drugs are being tested in COVID-19 patients across the nation, none have yet been proven safe and effective.
Vaccines typically aren’t used until all three phases are completed. Some in the medical community are speculating CCP virus vaccines could be used before then.
If vaccines have cleared early safety trials by the fall, they could be used “to ring-fence infections in cities, while we continue to study them to make sure they’re safe and effective for mass inoculation,” Dr. Scott Gottlieb, former commissioner of the Food and Drug Administration, said on CBS’s “Face the Nation” earlier on May 3.

Human Challenge Trials
One way to accelerate testing of vaccines is human challenge trials.In phase 3 trials, thousands of people volunteer to be injected, and researchers dose some with the vaccine candidate and others with a placebo. Researchers track the subjects for months to discern whether the vaccine is effective in preventing infection.
A human challenge trial would speed the process by adding a second step after the injections: deliberately infecting the volunteers with the CCP virus.
Taking into account the global pandemic, the authors argued in favor of considering human challenge trials as long as the participants were healthy and young.
“Multiple measures would be put in place to ensure that, prior to consenting, potential participants fully comprehend the unusual risks involved in the study,” wrote Nir Eyal, professor of bioethics at Rutgers University; Marc Lipsitch, an infectious disease epidemiologist at Harvard University; and Peter Smith, professor of Tropical Epidemiology at the London School of Hygiene & Tropical Medicine.
During the town hall, Trump said he supports the idea, saying volunteers would be aware of what was happening.
“They’re, in many cases, very good people. They want to help the process,” he said.
“The enormous human cost of the COVID-19 epidemic alters the optimization of the risk/benefit analysis,” they wrote.
Using challenge trials for CCP virus vaccines has faced opposition.
Myron Levine, a vaccine researcher at the University of Maryland School of Medicine, says he doesn’t believe such trials would actually be faster, given how quickly researchers are already moving on CCP virus vaccines.
“I cannot imagine that it would be ethical and would really speed up what we have to do,” he told Science Magazine.
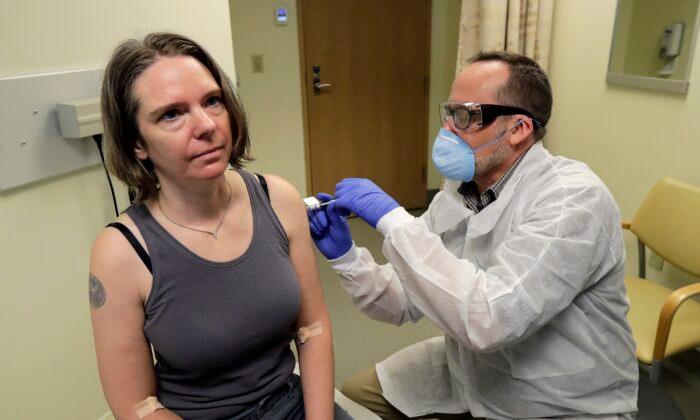



Friends Read Free