SPECIAL COVERAGE
Read More
Read More
Supreme Court Grapples With ‘Official’ vs. ‘Private’ Acts in Trump Immunity Appeal
The high court considers whether Trump is immune from prosecution in the federal election case in a decision that could delay his trials further into the year.
Supreme Court Grapples With ‘Official’ vs. ‘Private’ Acts in Trump Immunity Appeal
The high court considers whether Trump is immune from prosecution in the federal election case in a decision that could delay his trials further into the year.
Top Premium Reads
Top Stories
Most Read
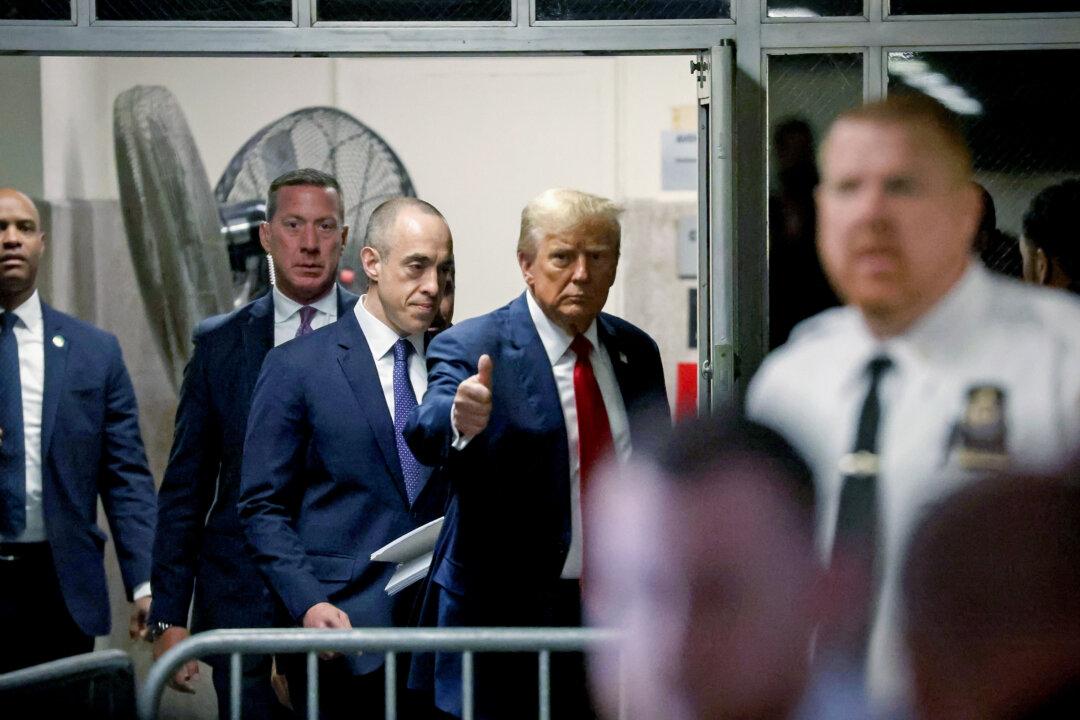
Ex-publisher Pecker Testifies About Deals Made in Trump Trial
Prosecutors charged Trump with 34 counts of falsifying business records, alleging a scheme to influence the 2016 elections.
Blinken Raises Concerns on Unfair Trade Practices in China
Blinken is on the second trip to China as secretary of state to tackle a series of contentious issues, including trade, human rights, and Russia’s Ukraine war.
House Judiciary Committee Launches Investigation Into Corporate Suppression of ‘Disfavored Platforms’
Coca-Cola and Orsted ’may be acting inconsistent with U.S. antitrust laws.’
House Lawmakers Introduce Legislation to Hold CCP Accountable for Genocide in China’s Xinjiang
‘Xi Jinping gave his henchmen the order to show no mercy,’ said Rep. Chris Smith.
‘Zero Emissions’ Freight: Biden Spending $1.5 Billion to Electrify US Trucking Industry
Transitioning commercial truck fleets requires up to $1 trillion in investment and will potentially push up freight rates, an expert warned.
Harvey Weinstein 2020 Rape Conviction Overturned by NY Appeals Court
Mr. Weinstein is currently serving a 23-year sentence in New York.
DOJ Requests Binance Founder Changpeng Zhao Receive 36 Months in Prison After Guilty Plea
DOJ is recommending that Changpeng Zhao, the founder of the world’s largest crypto exchange, Binance, serve 36 months in prison.
Biden Admin’s Former Disinformation Chief Accuses House Oversight Subcommittees of ‘Selectively’ Releasing Testimony
The Disinformation Governance Board was disbanded in 2022 after public concern over its ‘potential for censorship.’
US Economic Growth Slows to 1.6 Percent in 1st Quarter, Lowest Since 2022
Consumer and government spending slows.
A Peaceful Appeal for Freedom 25 Years Ago Still Echoes Today
About 10,000 people quietly congregated in Beijing on a spring day in 1999, now remembered as the April 25 appeal—the largest protest in China’s recent history.
Editors' Picks
Judge Unseals Documents Showing FBI Discussed ‘Loose Surveillance’ of Trump’s Plane
A large tranche of documents were unsealed by Judge Aileen Cannon on Monday, revealing the FBI’s code name for the probe.
Biden Heads to Syracuse to Tout New Investment in Chip Manufacturing
Micron will receive over $6 billion in government grants to boost domestic production.
Harvard Students Prop Up Tents to Protest Israel-Hamas War
Prime Minister of Israel Benjamin Netanyahu has called the ongoing pro-Palestinian protests across U.S. campuses ‘horrific.’
Trump Criticizes New York Judge After His Request to Attend Supreme Court Presidential Immunity Hearing Denied
A ruling on presidential immunity by the Supreme Court is expected by late June. However, initial oral arguments begin on Thursday.

Mahler’s ‘Resurrection’ Symphony: Answering Nihilism
Mahler’s “Resurrection” Symphony is the musical equivalent of “Hamlet.” What led to its creation?
Mahler’s ‘Resurrection’ Symphony: Answering Nihilism
Mahler’s “Resurrection” Symphony is the musical equivalent of “Hamlet.” What led to its creation?
Epoch Readers’ Stories
A History Of The American Nation
A patriotic poem by Ted Schneider
Of Cars and Kids
Why should our kids have to settle for a Trabant, or a Pyonghwa, education when they could have a BMW?
A Nation Divided
Poem by an American Patriot
What Is Going on Here?
There are two major things plants need to survive and continue generating our life saving oxygen. The first is CO2, and the second is sunshine.
Inspired Stories
Empower the World with Your Story: Share Love, Inspiration, and Hope with Millions
Special Coverage
Special Coverage

Goodbye, Double Chin: 3 Exercises for a Slimmer Neck
Mr. Rocky, an instructor of classical Chinese dance, shares three exercises to help you maintain a sleek neck.

Goodbye, Double Chin: 3 Exercises for a Slimmer Neck
Mr. Rocky, an instructor of classical Chinese dance, shares three exercises to help you maintain a sleek neck.

Art Awakens the Soul in Evelyn Waugh’s ‘Brideshead Revisited’
The 1945 novel traces the young man’s path from self-indulgence to a discovery of deeper meaning.

The Two Reputations of Robert E. Lee
While those in the past saw Lee’s stellar qualities, today, some cannot see that he was a man defined, like all of us, by his time.

Morality and Opera: A Traditional Message of Good Versus Evil
In this first installment of ‘A Modern Look at Opera,’ we are introduced to the strong moral messages in this classical performing art.

‘Terrestrial Verses’: Censorship Up Close and Personal
Iranians face absurd suppression of their rights by bureaucrats.

Robert Smalls: Navy Captain and Reconstruction-Era Politician
This former slave would not let anything stop him on the road to freedom.

Jedidiah Morse: Father of American Geography
In this installment of ‘Profiles in History,’ we meet a minister who possessed a keen interest in geography and a concern about Christian liberalism.

How the US–Iraq Relationship Devolved Into War
Decades of animosity swirl in Steve Coll’s ‘The Achilles Trap: Saddam Hussein, the C.I.A., and the Origins of America’s Invasion of Iraq.'

Art Awakens the Soul in Evelyn Waugh’s ‘Brideshead Revisited’
The 1945 novel traces the young man’s path from self-indulgence to a discovery of deeper meaning.

Are You Eating Real Food? The Slippery World of Food Fraud
This empire of deceit is valued at a staggering $50 billion a year.

These Texas Airports Have Flight Cancellation Rates That Place Them Among the Highest in the Country, Study Finds
Airports across the country have made the list with New York and New Jersery placing in the top 3.

Rocky Mountaineer Train Launches Summer Season Promising Spectacular Views
Rocky Mountaineer has four routes that will take passengers through Western Canada and the American Southwest.

After 5 Years of Closure, ‘Glamping’ Back Again in Yosemite National Park
Camping hopefuls can now enter a lottery to experience three of the five available campsites.
These Texas Airports Have Flight Cancellation Rates That Place Them Among the Highest in the Country, Study Finds
Airports across the country have made the list with New York and New Jersery placing in the top 3.




















































![[PREMIERING APR 26, 7:00PM ET] Hope for Israel | Special Report](/_next/image?url=https%3A%2F%2Fimg.theepochtimes.com%2Fassets%2Fthemes%2Feet%2Fimages%2FEET_default_700x420.jpg&w=1200&q=75)
















































































![[PREMIERING 4/25, 9PM ET] Up to 5 Years in Prison for Possession of a Meme? Hermann Kelly on Ireland’s New Hate Speech Bill](/_next/image?url=https%3A%2F%2Fimg.theepochtimes.com%2Fassets%2Fuploads%2F2024%2F04%2F25%2Fid5637157-240424-ATL_Hermann-Kelly_HD_TN-600x338.jpeg&w=1200&q=75)